A quick scroll through social media yields boundless aesthetic procedures, claiming to do everything from plump our lips to tighten our lids and purify our pores. While Internet-crowned “miracle fixes” might look convincing on the ’gram, often the real-life results are a whole different story.
“I think one advantage of social media is the ability to educate and present what’s available out there—but just because something is being promoted on social doesn’t mean it’s completely without risk or that results are guaranteed,” says New York City dermatologist Dr. Sejal Shah. “It’s important for people to really do their research.”
Here are five seen-everywhere tools and treatments that experts wish we’d stop wasting our time and money on—plus the hidden dangers that come with each.
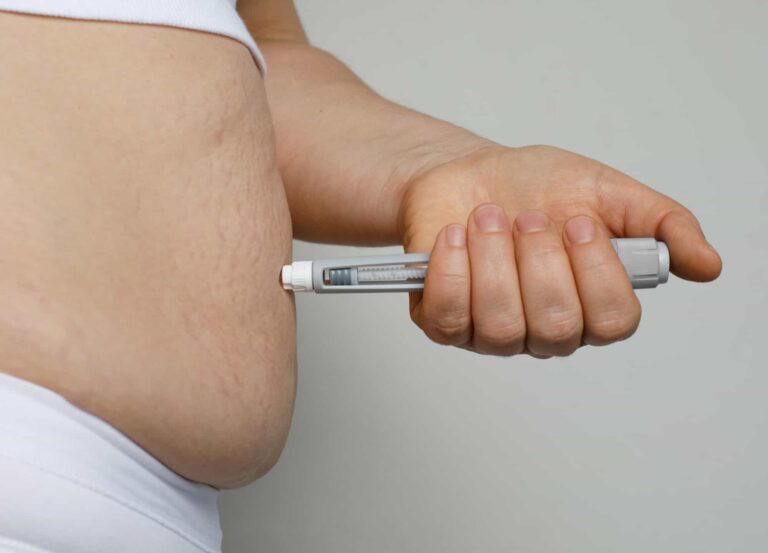
1. The don’t: hyaluron pen
Advertised as noninvasive and less painful than traditional filler injections, this buzzy pen-shaped device is designed to push plumping, moisture-magnet hyaluronic acid into the skin via “jet injection” rather than needles. Most hyaluron pens are DIY (often ordered online), although plenty of medical spas, aestheticians, and salon workers are touting the service as “needle-free lip injections” and the like.
FDA status
On October 8, 2021, the FDA issued a warning against the use of hyaluron pens by the public and health professionals, saying it “is aware of serious injuries and in some cases, permanent harm to the skin, lips, or eyes with the use of needle-free devices for injection of lip and facial fillers.” The FDA notes that it has not evaluated the safety and effectiveness of needle-free devices for injection of any dermal filler, nor has it approved the marketing of needle-free devices for injection of filler.
The administration recommends that consumers do not undergo any filler procedure performed with a needle-free device; do not buy or use any filler that is sold directly to the public; do not inject themselves or others using any device; and report any problems experienced after using needle-free devices to MedWatch, the FDA Safety Information and Adverse Event Reporting program.
The claim
Hyaluron pens are said to generate enough pressure to propel hyaluronic acid (and other materials) into the skin. “Many products claim that results can last up to 12 months—however, filler delivered this way is unlikely to penetrate beyond the top layers of the skin, and its effects will most likely be transient,” says Dr. Shah.
AmSpa describes the pen’s mechanism as “piercing” the skin with a jet of hyaluronic acid, not unlike the way flu vaccines are commonly administered. However, it notes, “even though these pens do not use needles, their use is considered the practice of medicine, and these procedures need to be performed by appropriate persons under medical supervision.”
Why it’s iffy
According to the FDA, “needle-free injection devices for aesthetic purposes do not provide enough control over where the injected product is placed. Lip and facial filler products sold directly to consumers online may be contaminated with chemicals or infectious organisms.”
Additional health risks include inflammatory skin reaction, hematomas (blood pooling under the skin), bacterial and fungal infections, skin discoloration, spreading of transmissible disease, and damage to eyes, due to excessive pressure. Dr. Shah adds that blood vessel occlusion is also a rare risk and that such blockages can lead to skin death and blindness.
On the hyaluron pen website, it’s repeatedly noted that, “Like with all injection procedures, complications can occur: bruising, swelling, occlusion, skin damage, Tyndall effect, necrosis, etc.”
Bottom line
“The idea of placing filler in micro boluses without a needle is a little bit absurd, to be honest,” says Los Angeles board-certified dermatologist Dr. Leonard Kim. “It takes years of training and experience to flow fillers into the correct areas of the dermis. It’s learning how to master this smooth flow and placement that makes a provider a good injector. Additionally, even qualified injectors have a difficult time injecting themselves.”
Beverly Hills, California, board-certified facial plastic surgeon Dr. Sagar Patel cautions against being sucked in by jaw-dropping visuals. “The companies marketing these products can use any before and after pictures and are not held to a code of ethics,” he notes. “So unfortunately, most users are sold on results that are not realistic.”
Related: Will Instagram’s New “Cosmetic Surgery Ban” Actually Change Anything?
2. The don’t: Kybella under-eye fat pad injections
Trending in Asia, this risky eye bag–deflating treatment is starting to gain traction in the U.S. Ever since Kybella (deoxycholic acid) won approval in 2015, RealSelf members have been asking about its potential for shrinking fat pads under the eyes.
FDA status
Off-label; not approved for this use. While Kybella enjoys a 77% Worth It Rating among RealSelf members, it’s approved solely for the treatment of a double chin. The FDA warns: “Avoid injecting in proximity to vulnerable anatomical structures.”
The claim
As a “cytolytic” drug that mimics bile-chewing stomach acid, Kybella destroys the membranes surrounding fat cells, causing their contents to spill out and be absorbed by the body. In theory, placement of Kybella directly into the puffy under-eye fat pads would achieve the same result it does under the chin: a flattering flatness.
Why it’s iffy
“The anatomy of the fat below the eyes is very complex,” says Dr. Patel. “The eyes are a very vascular space, with thin skin, multiple layers, and many important functional structures.” A lot can go wrong here, including nerve damage caused by excess swelling and even blindness, in very rare cases. “Kybella is known to cause a fair amount of swelling as the fat cells pop open and scavenger cells arrive to mop up,” explains Beverly Hills, California, dermatologist Dr. Ava Shamban. “This is no problem in the neck and, specifically, under the chin, but the eye is surrounded by fat pads, and the swelling could put pressure on the orbit, compromising the eyeball.” What’s more, she adds, “the optic nerve is encased in fat, and Kybella doesn’t discriminate, so the risk of nerve damage is very real.”
Bottom line
This technique “is very popular in Asia and Brazil,” notes Philadelphia dermatologist Dr. Nazanin Saedi. “I’m nervous about using Kybella under the eye, [because] the risk of vision-impairing side effects is so high.”
According to Dr. Lara Devgan, a plastic surgeon in New York City and the chief medical editor at RealSelf, Kybella “can effectively be used off label all over the body,” including for bra rolls and excessive fleshiness above the knees. But despite its success elsewhere, the potential for product migration makes placing it close to the eyes extremely risky.
Dr. Shamban notes that the reason Kybella tends to stay put under the chin is because it’s a flat, horizontal area. Since the under-eye area is more vertical, the product may travel, “and the destruction of fat cells could be realized in areas other than its specific target.” This potential migration “could cause an uneven hollowing effect [down the face] once the swelling subsides,” she says.
3. The don’t: semipermanent BB cream (aka “BB Glo”)
Covering a much larger stretch of real estate than, say, permanent eyeliner or lip color (procedures that contribute to an 80% Worth It Rating from RealSelf members), the new “BB Glo” is all about embedding light coverage and lasting brightness directly into the skin, as if you were slathered in your favorite complexion-perfecting BB cream 24/7, for six months to a year.
FDA status
Like tattoos, permanent makeup falls into the “cosmetic” category and is therefore not regulated by the FDA. However, that doesn’t mean the FDA doesn’t keep a watchful eye on this growing sector. This past May, it issued a safety advisory to consumers, tattoo artists, and retailers about inks tainted with microorganisms.
The claim
Over the course of one or more sessions, practitioners create tiny wounds in the skin via microneedling or microblading, then sink BB cream into the skin, just above the dermal layer. For the procedure to be considered cosmetic needling, practitioners “would need to be implanting pigment at less than 0.5 mm,” says Dr. Shamban. “However, in an unregulated industry such as this, there’s really no guarantee of this, and in some instances, even half a millimeter is too deep.”
Why it’s iffy
First off, this is not your typical BB cream from Sephora. “The spas I’ve found advertising [this] use either a cream formulated in Russia or one from Korea, which means the products don’t necessarily meet our typical [FDA-imposed] standards and their ingredient decks are potentially questionable,” says Dr. Shamban. Think: carcinogens, dyes, fragrances, allergens, and irritants—any of which, “if implanted into the skin at any level, can cause major problems,” she adds.
In addition to allergic reactions, risks include a range of infections, scarring, general irritation, and at the most extreme end of the spectrum, chronic allergic reaction and even potential skin cancer formation. “With cellular exposure to carcinogenic materials, skin cancer is a possibility, which may be due to the ability of the material to damage the genome or to disrupt healthy cellular metabolic processes,” Dr. Shamban explains.
Bottom line
“This process of implanting light or almost-white colored pigments into the dermis to brighten the skin is a definite don’t,” says Dr. Shamban. “Over time, the pigment particles may break down very unevenly, leaving permanent white blotches under the skin.”
While Dr. Shamban isn’t a fan of microblading as a whole, she urges those set on trying this particular trend to go above and beyond when researching qualified providers.
“Apparently there’s a BB Glow Academy [and similar programs], where aestheticians can get trained and certified in a single day. However, there’s no specific regulation around the procedure, nor are there true ‘experts’ in this field,” Dr. Shamban says. “Any spa owner or hairstylist can be trained in the procedure, neither of whom would presumably have the medical knowledge and understanding of the cellular physiology, dermal matrix, [or] specialized skin structures involved.” Consult with a board-certified dermatologist or other medical professional to fully discuss the risks and rewards.
Related: Deceptive Before & After Photos Are Everywhere. Here’s How to Spot the Red Flags.
4. The don’t: DIY blackhead removal
Blame the “popaholics” for the uptick in both classic metal blackhead removers and high-tech suctioning tools designed to vacuum out the goo that gums up our pores.
FDA status
Several blackhead extractors, including Imolake Blackhead Remover Pore Vacuum and Beautural Suction Blackhead Remover, decorate their packaging with claims of FDA approval. But when we asked the FDA to substantiate its involvement with these products, the agency revealed that neither of these companies is registered with the FDA. According to a spokesperson for the agency, “all companies must register with [the] FDA, regardless of what type of premarket review their product undergoes.” Other pore-clearing tools, including the new Rodan + Fields Pore Cleansing MD System device, define themselves as cosmetic products, thereby skirting FDA scrutiny.
The claim
After cleansing skin and prepping it with a hot towel to soften pores, pressure is applied to offending blackheads with the tool of one’s choice. These include specially designed tweezers, O-shaped comedone extractors, and vacuum-style suctioning wands. Manufacturers typically recommend following extraction with the application of a topical anti-acne solution.
Why it’s iffy
Not only can these devices be irritating, but they can potentially cause bruising, broken capillaries, stretching of the skin, and worsening of blackheads in the long-term. “Constantly manipulating the skin in this way actually enlarges pores, causing them to fill up faster,” says New York City dermatologist Dr. Shereene Idriss.
Bottom line
Leave extractions to the pros. “The blackhead tools popularized by Dr. Pimple Popper have become too easy to access online,” says Dr. Patel. “I’ve seen multiple fashion models with permanent scarring around their noses from these.”
5. The don’t*: plasma fibroblast skin-tightening therapy
Demand for plasma fibroblast skin-tightening, a process that creates controlled burning in the epidermis through tiny pulses of plasma gas, is seriously catching fire. “Over the past few years, the resurfacing technology has advanced to the point where plasma energy is harnessed in a handheld battery-operated pen,” explains New York City dermatologist Dr. Paul Jarrod Frank, who uses the Subnovii device in his practice on small facial areas, such as fine lines above the lips and on the forehead. However, in untrained hands—or with unapproved plasma devices—the procedure can be dangerous.
*Here’s where that little asterisk comes into play: the trend of black-market pens making their way into the hands of untrained practitioners has tainted this otherwise legit procedure. “I think because it’s not some big, noisy machine that costs $150,000, people think it’s easy and low-risk,” Dr. Frank says. “But it’s still a powerful device that, like a laser, can hurt somebody if misused.”
FDA status
While a slew of imitators is flooding the market, to date there is only one FDA-sanctioned plasma pen available: the Subnovii by Cartessa, which was approved just a few weeks ago, Dr. Frank says.
The rise of unlicensed plasma pens spurred Health Canada to issue a general warning to the public, noting, “Health Canada has not approved any plasma pens/fibroblast devices for sale in Canada. This means that these devices have not been evaluated for safety, effectiveness, or quality. Health Canada has advised the public, spas, and aestheticians to stop using plasma pens/fibroblast devices.”
The claim
The probes on these pen-like devices convert electrical energy to an electrically stimulated gas, which is pulsed just above the skin. “It almost looks like a little lightning bolt coming out of the pen,” Dr. Frank says. At approximately 1 mm above the epidermis, oxygen and nitrogen collide, producing plasma, which is pumped into the epidermis, causing thermal disruption (or burning) and eventual skin contraction.
But first, there’s a bit of crusting that has to happen—at least five days of downtime, during which the skin scabs over in a dot-like pattern (there’s no hiding it with makeup). “The brown dots are basically micro burns, which cause your skin to contract over time by stimulating collagen production,” Dr. Idriss explains.
Why it’s iffy
Plasma pens work their magic by burning the skin. Redness and irritation are the least of what could happen with a subpar practitioner.
“The risks associated with this device are exactly the same as with any form of laser resurfacing: excessive pigmentation, scarring, prolonged healing, and infection,” Dr. Frank explains. As such, plasma pen treatments should be offered only by licensed, board-certified dermatologists and plastic surgeons. “This is not an aesthetician device,” he adds.
Although physician assistants (PAs) and nurse practitioners (NPs) can legally perform this procedure in most states, Dr. Frank is the only one wielding the pen in his practice. “My patients expect my extensive experience in resurfacing for their treatments,” he says. “I believe extenders [like PAs and nurses] should only use this device for removal of benign lesions and very mild resurfacing with minimal downtime of a day or two.”
Bottom line
This treatment is more controversial than the previous four, and experts are split over the supposed benefits. (Patients are too: plasma pen has a 45% Worth It Rating on RealSelf, accompanied by some devastating reports of scarring, pigmentation issues, and skin texture changes.) While Dr. Shamban, Dr. Frank and Dr. Saedi say they’re happy to have access to this new technology, they also stress the very real risk consumers take by not carefully vetting their practitioners. “I’ve been trained on Subnovii plasma pen and love it,” says Dr. Saedi. “It can do amazing tightening, but it can also cause a lot of harm in the wrong hands.” According to Pasadena, California, facial plastic surgeon Dr. Kay Durairaj, “Anyone prone to hyperpigmentation or skin tone inconsistencies will be at higher risk for irritation and pigmentation from plasma pens.” She believes CO2 lasers and surgery to be the gold standard for skin tightening.